Foore Fucei liikme pikkus
Seni pole Eestis teadaolevalt ühtki ebaseaduslikult ehitatud hoonet kohtuotsuse alusel lammutatud. Kuidas sulle tundub praeguse Eesti koondise seis? Iga roll ja etendus on oma aja kontekstis. Kui seda esimest korda nägid, ütles kuulus loodusteadlane John Muir: " Kord aastas välja antava Sophie preemia asutas aastal Norra. Pille-Riin Tamsalus Tehnika 24, kpl.
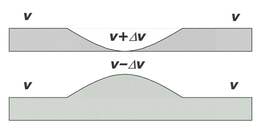
Villarreal, Seth A. Here, we present a crystallographic structure of the wild-type Synechococcus elongatus KaiB and a cryo-electron microscopy cryoEM structure of a KaiBC complex.
The crystal structure shows the expected dimer core structure and significant conformational variations of the KaiB C-terminal region, which is functionally important in maintaining rhythmicity.
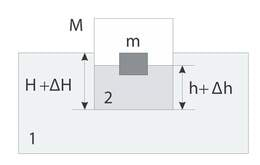
A KaiC mutation, RC, which has been shown to affect the affinity of KaiB for KaiC and lengthen the period in a bioluminescence rhythm assay, is found within the middle of the predicted KaiBC interface. We demonstrate here that the AV KaiC mutant sheds light on the former mechanism. It was previously reported that AV is less sensitive to dark pulse-induced phase resetting and has a reduced amplitude of the KaiC phosphorylation rhythm in vivo.

A maps to a loop loop that continues toward the phosphorylation sites. By pulling Foore Fucei liikme pikkus the C-terminal peptide of KaiC A-loopKaiA removes restraints from the adjacent loop whose increased flexibility indirectly promotes kinase activity.
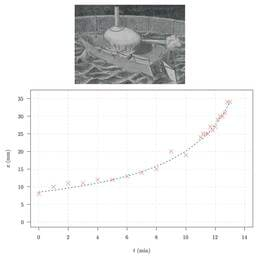
We found in the crystal structure that AV KaiC lacks phosphorylation at S and exhibits a subtle, local conformational change relative to wild-type KaiC.
Molecular dynamics simulations indicate higher mobility of the loop in the absence of the A-loop and mobility differences in other areas associated with phosphorylation activity between wild-type and mutant KaiCs.
Finally, the A-loop—loop relay that Foore Fucei liikme pikkus KaiC phosphorylation sites of KaiA dimer binding propagates to loops from neighboring KaiC subunits, thus providing support for a concerted allosteric mechanism of phosphorylation.